
The Principle of X-ray Fluorescence Spectrometry
- Ionization of an inner atomic shell (creation of an electron vacancy and emission of a photoelectron) gives rise to a highly excited short-lived atomic state liable to decay back to the ground state.
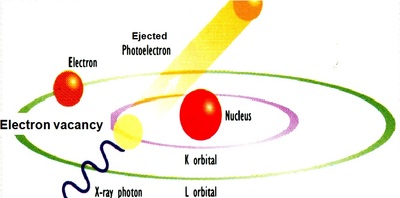
- Transfer an outer electron to fill the vacancy in the inner shell and the emission of an X-ray photon.
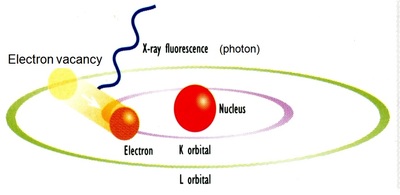
- The energies and wavelengths of the emission X-ray are characteristic (different) for each element which forms the foundation of the modern XRF Spectrometry.
© 2023 - LXCG London X-ray Consulting Group
Janesville, WI 53545
U.S.A.
info@LondonXrayConsultingGroup.com
Privacy Policy | Web Standards | Terms of Use | Accessibility
Key Topics:
- XRF Short Course
- Workshops - Sample Prep
- On-Site Training
- Meet the Team
- Contact Us

